After nearly three decades of development and three rounds of technological iteration, antibody-drug conjugates (ADCs) have become a mainstream class of anti-tumor therapeutics due to their high efficacy and favorable safety profile.
However, ADCs represented by Enhertu, which utilize thiol-based conjugation and high payloads, still exhibit on-target toxicities such as hematologic toxicity and interstitial lung disease. These side effects are largely attributable to premature payload shedding and the instability inherent in high drug-to-antibody ratios (DARs) characteristic of thiol-based conjugation.
Through advanced cell engineering and precise process control, we obtain homogeneous antibody Fc glycan structures. Building on this glycan foundation, we have developed a one-enzyme, two-step process to generate highly homogeneous DAR4 conjugates. Subsequently, by leveraging distinct glycan structures, we have also established a one-step transfer method for DAR2 conjugation. Glycan-specific conjugated ADCs exhibit superior hydrophilicity, serum stability, and pharmacokinetic properties, significantly enhancing their safety profile.
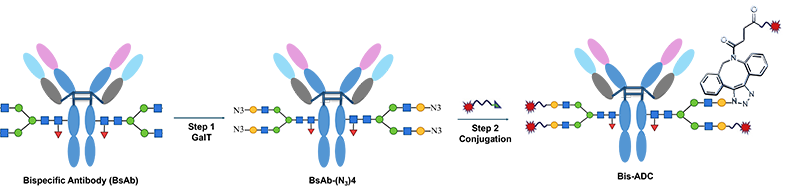
ADCs developed using this platform demonstrate significantly reduced toxicity, particularly hematologic toxicity. Representative candidate JSKN003* has multiple registrational clinical studies underway, while JSKN016* has completed dose optimization and dose confirmation and is poised to advance into Phase III clinical studies.
