Envafolimab(KN035)* is an independently developed subcutaneous PD-L1 single-domain antibody Fc fusion protein. It was approved for marketing in China in November 2021, for the treatment of unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) solid tumors.
Envafolimab efficiently blocks the binding of PD-1 and PD-L1, thereby activating an immune response against tumors. With a molecular weight half that of a conventional antibody, it exhibits enhanced tissue penetration and reaches tumor tissues via the lymphatic circulation system.
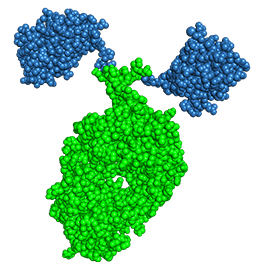
KN035 : Crystal Structure
Envafolimab offers significant advantages in convenience and compliance by avoiding intravenous infusion and can be administered in just 30 seconds, making it particularly suitable for frail, elderly, or IV-intolerant patients.
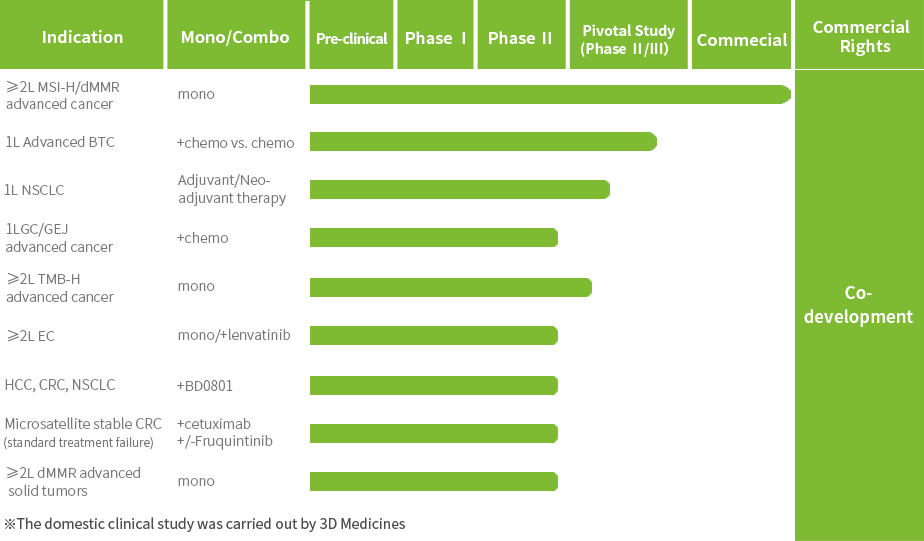
Multiple clinical trials for Envafolimab are currently underway to further expand its indications.The New Drug Application (NDA) for Envafolimab in combination with the Gemcitabine and Oxaliplatin (GEMOX) regimen for the first-line treatment of unresectable or metastatic biliary tract cancer (BTC) has been accepted by the National Medical Products Administration (NMPA).
Envafolimab has been granted three Orphan Drug Designations (ODD) by the U.S. Food and Drug Administration (FDA) for the treatment of advanced biliary tract cancer, soft tissue sarcoma and gastric cancer and gastroesophageal junction cancer; it has been granted Breakthrough Therapy Designation (BTD) by the NMPA for the treatment of unresectable or metastatic solid tumors with high tumor mutation burden (TMB-H).
ASCO 2020 poster’╝ÜEnvafolimab (KN035) in advanced tumors with mismatch-repair deficiency
ASCO 2019 poster’╝ÜPhase I Safety and Pharmacokinetic Study of KN035, the first subcutaneously administered, novel fusion Anti-PD-L1 Antibody in Japanese Patients with Advanced Solid Tumors
ASCO 2019 poster’╝ÜPhase I Study of KN035, the first subcutaneously administered, novel fusion Anti-PD-L1 Antibody in Patients with Advanced Solid Tumors in China
ESMO 2018 poster’╝ÜPhase I Study of KN035, a novel fusion anti-PD-L1 antibody administered subcutaneously in Patients with Advanced Solid Tumors in the USA
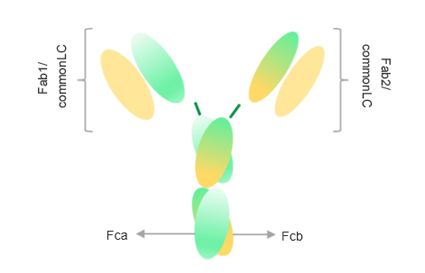
Anbenitamab (KN026)* is an anti-HER2 bispecific antibody developed using the proprietary Fc-based heterodimer bispecific platform technology called CRIB (Charge Repulsion Induced Bispecific). Anbenitamab can simultaneously bind two non-overlapping epitopes of HER2, resulting in HER2 signal blockade. Through antibody-induced receptor clustering, it enhances ADCC and CDC effects while promoting the down-regulation of HER2 receptors on the cell surface.
KN026 : Crystal Structure
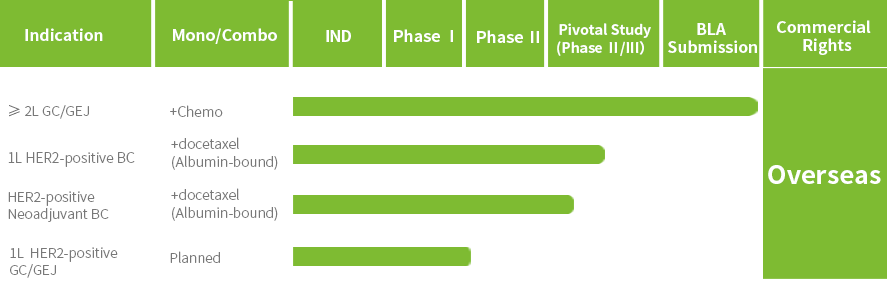
In September 2025, the first New Drug Application (NDA) for anbenitamab injection has been accepted by the National Medical Products Administration (NMPA) for the treatment of HER2-positive gastric cancer (GC). Currently, several pivotal clinical trials of KN026 for second-line or above HER2-positive GC/gastroesophageal junction cancer (GEJ), first-line HER2-positive breast cancer (BC), neoadjuvant treatment of HER2-positive BC are being conducted.
Anbenitamab has been granted Orphan Drug Designation by the U.S. Food and Drug Administration (FDA) for the treatment of HER2-positive or low expressing GC; it has been granted Breakthrough Therapy Designation by NMPA for the treatment of patients with HER2-positive GC/GEJ who have failed first-line standard treatment.
SABCS 2023 poster’╝ÜTwo and a half years follow-up data of HER2-targeted bispecific antibody KN026 combined with docetaxel as first-line treatment for HER2-positive recurrent/metastatic breast cancer
ESMO 2023 poster’╝ÜKN026 in combination with docetaxel as neoadjuvant treatment for HER2+ early or locally advanced breast cancer (BC): A single arm, multicenter, phase 2 study
SABCS 2022 poster’╝ÜEfficacy and safety results of KN026, a HER2-targeted bispecific antibody combined with docetaxel in first-line treatment of HER2-positive recurrent/metastatic breast cancer
SABCS 2022 poster’╝ÜKN026 in combination with docetaxel as neoadjuvant treatment for HER2-positive early or locally advanced breast cancer: A single arm, multicenter, phase 2 study
ASCO 2022 poster’╝ÜA phase II study evaluating KN026 monotherapy in patients with previously treated, advanced HER2-expressing gastric or gastroesophageal junction cancers
ASCO 2021 Abstract’╝ÜThe Preliminary Efficacy of KN026 (Anti-HER2 BsAb) in Advanced Gastric and Gastroesophageal Junction Cancer Patients with HER2 Expression
AACR 2020 poster’╝ÜUsing translational tumor growth inhibition modeling approach and population PK analysis to predict efficacious doses for KN026, a HER2 bispecific antibody
ASCO 2020 poster’╝ÜPreliminary Safety, Efficacy and Pharmacokinetics (PK) Results of KN026, a HER2-targeted Bispecific Antibody in Patients (pts) with HER2-positive Metastatic Breast Cancer
Anbenitamab repodatecan (JSKN003) is developed by site-specific conjugation to the Fc glycans of anbenitamab (KN026), resulting in a homogeneous and stable ADC with a drug-to-antibody ratio (DAR) of 4. JSKN003 binds to two HER2 epitopes on tumor cells and release topoisomerase I inhibitors through cellular endocytosis, exerting anti-tumor effects.
Compared to similar ADCs, JSKN003 demonstrates better serum stability, reduced hematological toxicity, and stronger tumor inhibition and bystander effect, resulting in significantly wider therapeutic window.
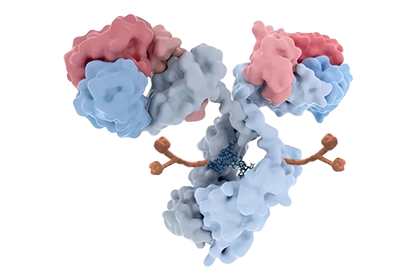
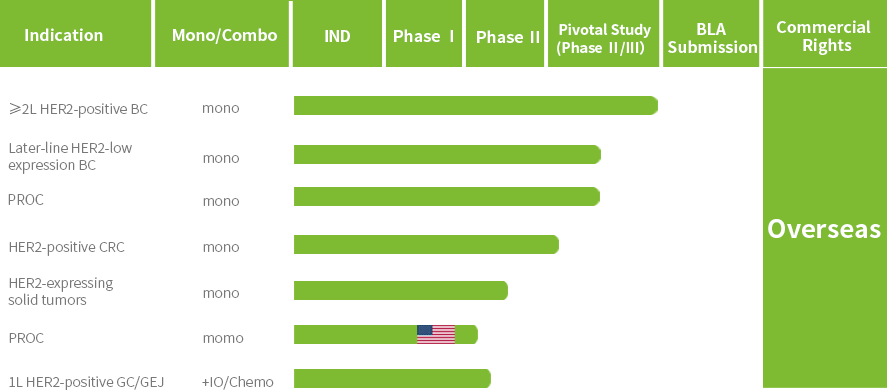
Patient enrollment has been completed for the Phase III clinical trial of JSKN003 in second-line HER2-positive breast cancer (BC), and a New Drug Application (NDA) is expected to be submitted in 2026. Additionally, several other registrational studies are ongoing, including trials in all-comer platinum-resistant ovarian cancer (PROC), HER2-low BC, and HER2-positive colorectal cancer (CRC).
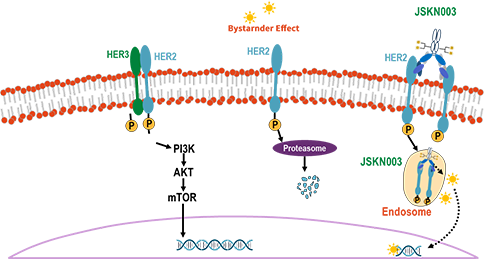
JSKN003 has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Administration (FDA) for gastric cancer (GC) and gastroesophageal junction cancer (GEJ), has been granted Fast Track Designation (FTD) by the FDA for the treatment of advanced or metastatic platinum-resistant recurrent epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer (PROC), not restricted by HER2 expression, has been granted Breakthrough Therapy Designation (BTD) by the FDA for the treatment of patients with HER2-expressing PROC who have received prior treatment with bevacizumab; It has also been granted two BTDs by the National Medical Products Administration (NMPA) for PROC and HER2-positive advanced CRC that has failed prior oxaliplatin, fluorouracil, and irinotecan therapy.
ESMO 2025 poster’╝ÜBiparatopic anti-HER2 antibody drug conjugate (ADC) JSKN003 in the treatment of primary platinum-refractory ovarian cancer (OC)
ESMO 2025 poster’╝ÜEfficacy and Safety of JSKN003, a Biparatopic anti-HER2 Antibody Drug Conjugate (ADC), in Patients with HER2-positive Metastatic Colorectal Cancer (mCRC)
ASCO 2025 poster’╝ÜJSKN003, a biparatopic anti-HER2 antibody drug conjugate (ADC), in the treatment of platinum-resistant ovarian cancer (PROC): Updated findings from two clinical trials
ASCO 2025 poster’╝ÜJSKN003, a biparatopic HER2-targeting ADC, in heavily pretreated HER2-positive breast cancer: A pooled analysis of early-phase studies
ASCO 2025 poster’╝ÜA pooled analysis of JSKN003, a biparatopic anti-HER2 antibody conjugate (ADC), in patients with advanced HER2-overexpressing (IHC 3+) gastrointestinal tumors
ESMO 2024 poster’╝ÜJSKN003, a HER2-targeting antibody-drug conjugate, in patients with platinum-resistant ovarian cancer: A pooled analysis of two studies
ESMO 2024 poster’╝ÜEvaluation of the safety and efficacy of JSKN003 in patients with advanced HER2-positive (IHC 3+) solid tumors (excluding breast cancer)
ASCO 2024 poster’╝ÜEvaluation of the safety, pharmacokinetics, and efficacy of JSKN003 in patients with advanced solid tumors: A phase I/II clinical study
AACR 2024 poster’╝ÜSafety and efficacy of JSKN003 in patients with advanced/metastatic solid tumors: A first-in-human, dose-escalation and expansion, multicenter, open-label, phase I study
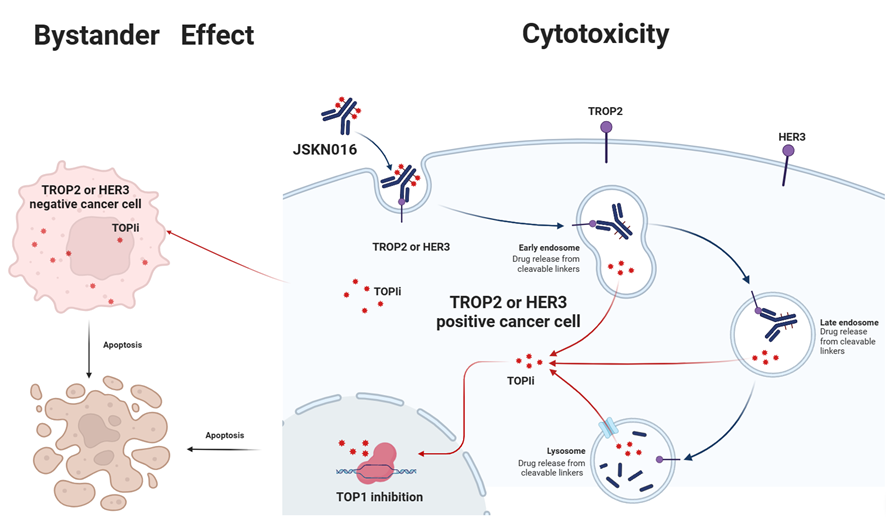
JSKN016 is a TROP2/HER3 targeting bispecific ADC developed using the proprietary single-domain antibody and bispecific antibody platforms. It is conjugated via site-specific glycosylation to generate a homogeneous and stable ADC with a drug-to-antibody ratio (DAR) of 4.
JSKN016 binds to TROP2 and/or HER3 on tumor cells and release topoisomerase I inhibitors through cellular endocytosis, exerting anti-tumor effects.
JSKN016 has demonstrated promising antitumor activity and a favorable safety profile across multiple solid tumors. Dose optimization and dose confirmation have been completed, and it is poised to advance into Phase III clinical studies.
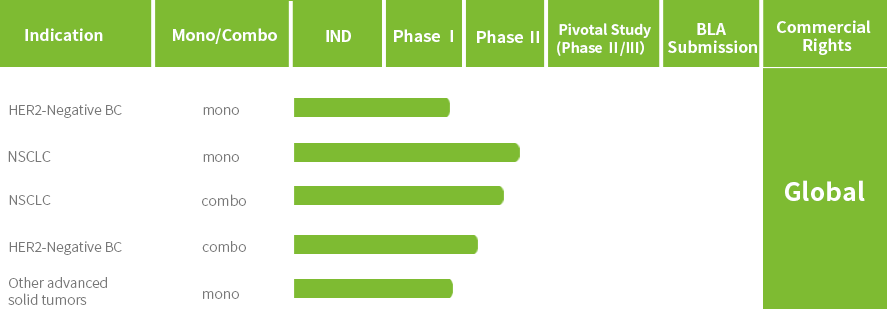
JSKN033 is an independently developed high-concentration subcutaneous formulation, combining the ADC (JSKN003)* with PD-L1(Envafolimab)* .
JSKN033 combines the benefits of immunotherapy and ADCs while enhancing safety and convenience through subcutaneous administration.
JSKN033 is currently undergoing Phase I/II clinical trials in China and Australia for the treatment of solid tumors, and a Phase II study for HER2-mutant/expressing non-small cell lung cancer is also underway. The IND application for the Phase II clinical study of JSKN033 in combination with chemotherapy as first-line treatment for advanced cervical cancer has been accepted by the CDE.
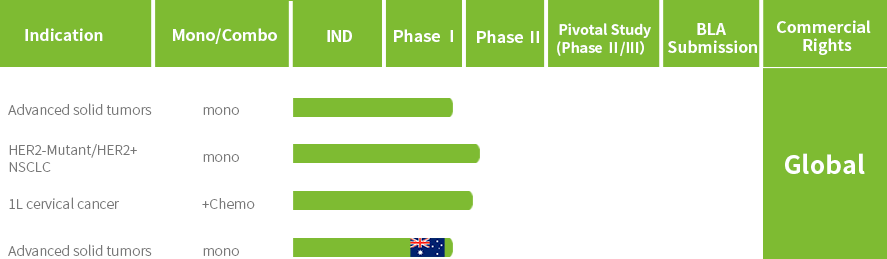
JSKN022 is a bispecific single-domain antibody targeting both PD-L1 and integrin αvβ6, developed through antibody-directed evolution. It is conjugated via site-specific glycosylation to generate a homogeneous and stable ADC with a drug-to-antibody ratio (DAR) of 4.
Upon binding to PD-L1 and/or integrin αvβ6 on the surface of tumor cells, JSKN022 can release topoisomerase I inhibitors through cellular endocytosis, exerting anti-tumor effects. Additionally, JSKN022 blocks the binding of PD-L1 and PD-1, thereby activating antitumor immune responses. Simultaneously, by blocking integrin αvβ6, it inhibits the production of TGFB 1/3 and modulates the tumor immune microenvironment.
The Phase I clinical study of JSKN022 has been conducted in China.

JSKN027 is a first-in-class bispecific ADC designed to co-engage PD-L1 and VEGFR2. By leveraging glycan-specific conjugation technology, it precisely links its cleavable linker and topoisomerase I inhibitor payload to the antibody’s Fc region - maintaining a strong safety profile while unlocking potent anti-tumor activity.
JSKN027’s efficacy stems from a unique three-fold synergistic mechanism. Beyond the standard ADC effects of targeted cell killing and bystander activity, it also inhibits tumor angiogenesis by blocking VEGF/VEGFR2 signaling and reverses immune suppression by disrupting the PD-1/PD-L1 checkpoint. This integrated attack enhances overall anti-tumor power and is anticipated to help overcome therapeutic resistance. As a result, JSKN027 is poised to offer a novel and robust treatment avenue for patients across multiple solid tumor types.
The IND application for the phase I clinical study of JSKN027 for the treatment of advanced malignant solid tumors has been accepted by CDE.

KN019 is a biosimilar of Belatacept (Nulojix®).
KN019 has completed Phase II clinical study with positive results. In November 2023, KN019 received clinical trial approval from the National Medical Products Administration (NMPA) for its subcutaneous formulation.

KN046 is an independently developed PD-L1/CTLA-4 bispecific antibody, engineered to target the tumor microenvironment with high PD-L1 expression, and Treg (suppress tumor immunity) clearing function.
Multiple clinical trials of KN046 are ongoing in China, the U.S., and Australia.
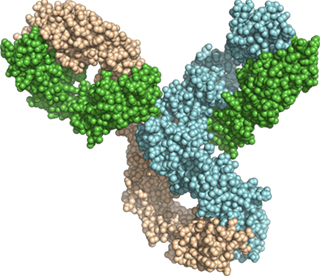
KN046 : Crystal Structure
KN046 has been granted Orphan Drug Designation by the U.S. Food and Drug Administration (FDA) for the treatment of thymic epithelial tumors.
ESMO 2023 poster’╝ÜKN046 in Patients with ≥2L R/M Thymic Carcinoma: A Prospective, Single-arm, Multi-center, Phase 2 Study
ESMO 2023 poster’╝ÜThe preliminary data from a single-arm, open-label, multicenter phase 2 clinical trial’╝ÜKN046 combined with axitinib as first-line (1L) treatment for NSCLC
ESMO 2022 poster’╝ÜA phase II study of KN046 (an anti-PD-L1/CTLA-4 bispecific antibody) in patients with metastatic non-small cell lung cancer (NSCLC) who failed to prior EGFR-TKIs treatment
ESMO 2022 poster’╝ÜA phase II study of KN046 (an anti-PD-L1/CTLA-4 bispecific antibody) in patients with metastatic non-small cell lung cancer (NSCLC) who failed in the first-line treatment
ESMO 2022 poster’╝ÜTwo-year follow-up from KN046 in combination with Platinum doublet chemotherapy as first-line’╝ł1L’╝ē treatment for NSCLC: an open-label, multi-center phase 2 trial
WCLC 2020 Mini oral report’╝ÜPreliminary safety, efficacy results of KN046 (bispecific anti-PD-L1/CTLA4) in subjects with rare thoracic tumors
WCLC 2020 poster’╝ÜA phase II study of KN046 (a bispecific anti-PDL1/CTLA-4) in patients (pts) with metastatic nonsmall cell lung cancer (NSCLC)
ASCO-GI 2020 poster’╝ÜThe preliminary efficacy and safety of KN046 plus concurrent chemoradiation therapy in recurrent and metastatic esophageal squamous cell carcinoma
ASCO 2020 poster’╝ÜThe preliminary efficacy and safety data of KN046 (bispecific anti-PD-L1/CTLA4) in patients failed on prior immune checkpoint inhibitors therapy
ASCO 2019 poster’╝ÜPreliminary safety, efficacy and pharmacokinetics (PK) results of KN046 (bispecific anti-PD-L1/CTLA4) from a first-in-human study in subjects with advanced solid tumors
