Bispecific antibodies can simultaneously recognize and bind two distinct antigens or epitopes. enabling flexible target design to block multiple signaling pathways and enhance tumor targeting and immune effector functions (e.g., antibody-dependent cell-mediated cytotoxicity [ADCC] and complement-dependent cytotoxicity [CDC]).
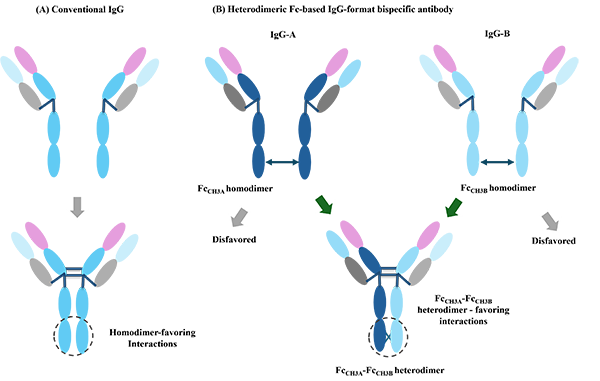
We have developed bispecific antibodies via multiple engineering approaches: i) Fc-based heterodimeric bispecific antibodies using the Charge Repulsion Induced Bispecific (CRIB) platform, which effectively addresses CMC challenges in bispecific antibody development. Antibodies generated from this platform are highly similar to natural antibodies in structure, shape, and molecular size. ii) Symmetrical tetravalent bispecific antibodies* developed using single-domain antibody combinations. iii) Two-in-one antibodies developed through antibody directional evolution, including both traditional antibody formats and single-domain antibodies. Combinations of these methods can further generate multifunctional antibody drugs.
Multiple bispecific antibodies or bispecific ADCs from our pipeline have entered clinical studies. The first New Drug Application (NDA) for the representative product, Anbenitamab (KN026)* , was accepted by the NMPA in September 2025.
* Wei H, Cai H, Jin Y, et al. Structural basis of a novel heterodimeric Fc for bispecific antibody production. Oncotarget, 2017, 8(31): 51037.
